Using Folic Acid Supplements in Fertility Treatments
In my current series of preconception webinars, I am not surprised by the number of women joining me who have had multiple miscarriages or who are currently having IVF (often unsuccessfully). They didn’t know they had the MTHFR gene and even after multiple pregnancy losses, still were not checked for the gene. Eventually, they sought the answer themselves.
Folic Acid Supplements in Fertility Treatments
Folate is critical for DNA methylation and cell division. As a result, it’s also important for the proper development of ova, or egg cells, that can successfully implant in the uterus.
But when we say folate what do we mean?
Well, think of ‘folate’ as an umbrella term. Under that umbrella, there is folic acid (the synthetic man made form), folinic acid which is an important cofactor for healthy DNA creation, and our active folate, called5-MTHF (methyltetrahydrofolate).
In IVF and fertility issues, doctors and specialists alike are still recommending a high dose 5mg folic acid supplement to remedy a MTHFR mutation.
We also have mandatory folic acid supplementation in all our commercial bread and in many of our breakfast cereals, juices, protein bars, shakes, and energy drinks. So researchers are now looking into what these high doses are doing to us.
You all know that those with the MTHFR gene mutation are counseled into avoiding folic acid. The reason is that folic acid has the potential to build up and inhibit our DHFR enzyme, which is crucial in our folate pathway. Some research has also shown that unmetabolized folic acid builds up and affects our immune system negatively too.
But now we know more! Some really interesting studies on folic acid were released in the latter half of 2015 that specifically focused on folic acid. These studies looked at the 5mg dose of folic acid to see how it affected fertility. Now, this is interesting to me, because many of our patients in the clinic have been prescribed 5mg of folic acid in response to their doctor seeing a MTHFR C677T homozygous result, or to prepare their body before they start IVF cycles.
So let’s take a closer look:
We know that fertility is decreasing world wide and we have to ask ourselves why. Sure we have a more toxic environment than ever before; yes we are more stressed than previous generations and have worse diets and fewer nutrients in our soil. But a recent study completed in the Human Molecular Genetic Journal at the end of 2015 showed some interesting information. They looked at DNA methylation of the sperm when they gave folic acid. The researchers trialed 5mg of folic acid in infertile men and acknowledged that serum folate concentrations increased significantly after 6 months of folic acid supplementation. They also noticed a slight, but non-significant increase in sperm numbers, but the surprising thing is that they found a ‘significant loss of methylation across the sperm epigenome’, and more so if you were homozygous for the MTHFR C677T mutation.
What is more alarming is that the researchers suggested that this loss of methylation in sperm DNA might be transmitted to the offspring. So what they are saying is that folic acid at high doses not only decreases the fertility of men by negatively affecting their DNA, but also that these effects may be passed onto the child.
Is this significant? Could this be adding to the infertility effect we are seeing in men?
In the Clinical Journal of Nutrition in 2015, Karen Christensen found that high folic acid consumption reduces MTHFR protein and activity levels, creating a pseudo MTHFR deficiency in mice.This deficiency affects liver cells ability to metabolize fat and affects cell membrane integrity. That’s why we often see elevated cholesterol levels in people with MTHFR deficiency and issues with egg integrity in women undergoing IVF.
Another study looking at folic acid supplementation in people with the MTHFR gene going into IVF also revealed some interesting data. This Swedish study found that the higher the folic acid intake, the higher the plasma folate. Overall, the conclusion was that ‘high folic acid intake did not seem to assist infertile women to achieve pregnancy after fertility treatment’.
It’s the same result as above for the men.
So we seem to be getting more of the same. Yes, folic acid is going to increase serum folate levels, that much makes sense. However, it does not help DNA methylation, that’s why it’s not helping fertility.
Methylation of DNA is what controls our fertility. Just because we’ve always done something doesn’t make it right. There should be a worldwide review of folic acid supplementation in fertility treatment. There is enough research emerging to have a review of existing protocols.
Find out more about the study.

Founder MTHFR Support Australia
What Is Glyphosate And How Can It Affect Our Health
Roundup is a popular brand of herbicide by Monsanto and has been making rounds not because of how great a product it is but because of its active ingredient: Glyphosate, a compound declared carcinogenic by the International Agency for Research on Cancer or IARC.
It’s household popularity is not just what makes it dangerous. It made headlines when people found out Roundup was being used on GMOs (Genetically Modified crops). Research has also linked it to antibiotic resistance and hormone disruption. Because of this, many governments are considering banning or at least restricting the use of the substance particularly in public places such as school districts.
Glyphosate use
Monsanto introduced Glyphosate in the US in 1974 and was originally used to kill weeds by blocking proteins essential to plant growth. It’s now a common herbicide in more than 160 countries with a reported use of at least 1.4 billion lbs. (635 million kg) annually. While this is mostly sold for home use, it’s mostly applied by the agricultural sector on corn, soy, and cotton crops, especially in the US.
Its use skyrocketed after seeds were genetically engineered to tolerate the chemical. Because these seeds produce plants that are not killed by glyphosate, farmers can apply the weed killer to entire fields without worrying about destroying crops.
The effect of Glyphosate to our health
Glyphosate has been linked to the following diseases/conditions:
1. Gastrointestinal disease
- The action of glyphosate as an antibiotic may alter the gastrointestinal microbiome in vertebrates, which could favor the proliferation of pathogenic microbes in humans, farm animals, pets and other exposed vertebrates. Basically, by destroying the Shikamate pathway in plants (and we eat these plants, and animals feed on them) our gut microbiota is affected and we are seeing a reduction in Lactobacillus among other things.
- Glyphosate-contaminated soybean feeds used in the pork industry have also been associated with elevated rates of gastrointestinal-health problems and birth defects in young pigs. Related impacts have been observed in poultry.
2. Obesity
- It has been proposed that the exponential increase in the production of synthetic organic and inorganic chemicals may be causal in the current worldwide obesity epidemic, due to alterations in body chemistry that promote weight gain.
- The shikimate pathway is the route for aromatic amino acids phenylalanine, tyrosine and tryptophan. Tryptophan is an essential amino acid, meaning that mammalian cells cannot synthesize it. Serum tryptophan depletion leads to serotonin and melatonin depletion in the brain. Since serotonin (derived from tryptophan) is a potent appetite suppressant, it follows that serotonin deficiency would lead to overeating and obesity.
- As we have seen, tryptophan supplies could be depleted both in plant-based food sources and through impaired tryptophan synthesis by gut bacteria as direct effects of glyphosate. The observed 20-fold increase in the synthesis of tryptophan-derived polyphenolic flavonoids in the context of glyphosate provides strong evidence of impaired tryptophan synthesis.
3. Cobalamin deficiency
- Species of Lactobacillus and Bifidobacterium have the capability to biosynthesize folate, so their disruption by glyphosate could contribute to folate deficiency. Either cobalamin (B12) or folate deficiency leads directly to impaired methionine synthesis from homocysteine, because these two vitamins are both required for the reaction to take place. This induces hyperhomocysteinemia (high homocysteine). Because a deficiency in cobalamin can generate a large pool of methyl-tetrahydrofolate that is unable to undergo reactions, cobalamin deficiency will often mimic folate deficiency.
- We depend on our gut bacteria to produce cobalamin, and impaired cobalt supply would obviously lead to reduced synthesis of this critical molecule. Glyphosate is known to chelate or bind to metals like iron, copper, cobalt, molybdenum, zinc, and magnesium. It has also been proposed that chelation by glyphosate of both cobalt and magnesium contributes to the impaired synthesis of aromatic amino acids in Escherichia coli bacteria. Thus, it is plausible that glyphosate similarly impairs cobalamin function in humans by chelating cobalt. In addition is severely affecting our zinc, molybdenum, iron, copper, manganese, and calcium in foods and the body.
4. Cancer
- Despite most studies saying it’s generally not cancerous, one study on breast cancer conducted by Thongprakaisang S et. al. confirms at least one connection.
- Glyphosate exerts proliferative effects only in human hormone-dependent breast cancer, T47D cells, but not in hormone-independent breast cancer. The proliferative concentrations of glyphosate that induced the activation of estrogen response element transcription activity were 5-13 fold of control in T47D-KBluc cells.
- This activation was inhibited by an estrogen antagonist, ICI 182780, indicating that the estrogenic activity of glyphosate was mediated via ERs. This indicates both low and environmentally relevant concentrations of glyphosate possessed estrogenic activity.
- Recently Stephanie Seneff (the world’s most significant researcher of Glyphosate) released a paper discussing the link between glyphosate and cancer. In this paper she says “glyphosate has a large number of tumorigenic effects on biological systems, including direct damage to DNA in sensitive cells” she goes on to say that “epidemiological evidence supports strong temporal correlations between glyphosate usage on crops and a multitude of cancers that are reaching epidemic proportions, including breast cancer, pancreatic cancer, kidney cancer, thyroid cancer, liver cancer, bladder cancer and myeloid leukaemia”
5. Infertility
- Glyphosate has been described as an endocrine disruptor affecting the male reproductive system.
- Glyphosate toxicity was proposed to implicate CA(+2) overload, cell signaling misregulation, stress response of the endoplasmic reticulum, and/or depleted antioxidant defenses, could contribute to Sertoli cell disruption in spermatogenesis that could have an impact on male fertility.
6. Kidney disease
- The strong association of the consumption of hard water and occurrence of chronic kidney disease has been subjected to many discussions among investigators, but none of the available theories could explain this relationship coherently.
- Glyphosate is metabolized via the glyoxalate pathway. When this pathway is inundated more oxalate is formed and this is a direct cause of kidney disease. Oxalates are naturally occurring substances found in a wide variety of foods and although they play a supportive role in the metabolism of many plants and animals when they over accumulate inside our body, they can form stones in the kidney. They bind to calcium and can also interfere with calcium metabolism.
What now?
The EPA is reviewing its approved uses of glyphosate while countries such as Sri Lanka has banned it. Brazil is considering a similar move. Mexico and the Netherlands have imposed new restrictions, and Canada has just begun a process to consider new rules.
As for Australia, it may take a while for our government to follow suit .
So what can we do?
- Avoid genetically modified foods
- Eat as organically as you can
- Avoid canola oil
- Avoid processed foods
- Regularly check key nutrients that may be depleted by glyphosate – copper, zinc, iron, manganese, magnesium, cobalt (B12) and sulfur
- If you have a family history of kidney stones or gall bladder issues consult someone who knows about oxalates and glyphosate to get yourself assessed.
- If you have a family history of thyroid disease, you may have oxalate issues
- If you have gut issues, particularly low lactobacillus you may have oxalate/glyphosate issues.
- If you have recurrent diarrhea you may have oxalate issues.
- If you are juicing lots of high oxalate foods like spinach, beetroot, carrot, and celery these may disturb kidney function. My motto is ‘if you can’t eat it on a plate then don’t juice it. That means that if the amount you juiceis too much for you to sit down and eat at the one meal, you are having too much.
- I suspect those with chronic iron issues may have an oxalate/glyphosate problem. Because oxalates will bind to iron making it unavailable.
- DEFINITELY DON’T USE ROUNDUP AT HOME!!!!!
If you are preparing your body for a healthy and thriving pregnancy, we have a free pregnancy checklist of the “10 most important things you need to do before you become pregnant”
References
MYERS JP, ANTONIOU MN, BLUMBERG B, ET AL. CONCERNS OVER USE OF GLYPHOSATE-BASED HERBICIDES AND RISKS ASSOCIATED WITH EXPOSURES: A CONSENSUS STATEMENT. ENVIRON HEALTH. 2016;15:19.
BAILLIE-HAMILTON PF. CHEMICAL TOXINS: A HYPOTHESIS TO EXPLAIN THE GLOBAL OBESITY EPIDEMIC. J ALTERN COMPLEMENT MED. 2002;8(2):185-92.
BREISCH ST, ZEMLAN FP, HOEBEL BG. HYPERPHAGIA AND OBESITY FOLLOWING SEROTONIN DEPLETION BY INTRAVENTRICULAR P-CHLOROPHENYLALANINE. SCIENCE. 1976;192(4237):382-5.
ZHAO J, WILLIAMS CC, LAST RL. INDUCTION OF ARABIDOPSIS TRYPTOPHAN PATHWAY ENZYMES AND CAMALEXIN BY AMINO ACID STARVATION, OXIDATIVE STRESS, AND AN ABIOTIC ELICITOR. PLANT CELL. 1998;10(3):359-70.
ROSSI M, AMARETTI A, RAIMONDI S. FOLATE PRODUCTION BY PROBIOTIC BACTERIA. NUTRIENTS. 2011;3(1):118-34.
THONGPRAKAISANG S, THIANTANAWAT A, RANGKADILOK N, SURIYO T, SATAYAVIVAD J. GLYPHOSATE INDUCES HUMAN BREAST CANCER CELLS GROWTH VIA ESTROGEN RECEPTORS. FOOD CHEM TOXICOL. 2013;59:129-36.
JAYASUMANA C, GUNATILAKE S, SENANAYAKE P. GLYPHOSATE, HARD WATER AND NEPHROTOXIC METALS: ARE THEY THE CULPRITS BEHIND THE EPIDEMIC OF CHRONIC KIDNEY DISEASE OF UNKNOWN ETIOLOGY IN SRI LANKA?. INT J ENVIRON RES PUBLIC HEALTH. 2014;11(2):2125-47.
SAMSEL, A., & SENEFF, S. (2015). GLYPHOSATE, PATHWAYS TO MODERN DISEASES IV: CANCER AND RELATED PATHOLOGIES. JBPC JOURNAL OF BIOLOGICAL PHYSICS AND CHEMISTRY, 15(3), 121-159. DOI:10.4024/11SA15R.JBPC.15.03
What’s All The Fuss About Homocysteine?
What can you do to make sure you homocysteine does not become a problem? If homocysteine is a problem, what do you do?
- First, we need to gain an awareness of how the body uses and metabolizes homocysteine.
- Second, you will want to ensure you are getting enough B vitamins, folate and trimethylglycine (TMG).
B vitamins, folate and TMG all work in the body as cofactors with enzymes. Enzymes need specific cofactors bound to them in order for them to function. Vitamin cofactors make the metabolizing homocysteine possible. It does not matter if you have mutations in enzymes such as methylenetetrahydrofolate reductase (MTHFR) or beta-homocysteine S-methyltransferase (BHMT) that reduce your body’s capacity to metabolize homocysteine. If your body’s enzymes do not have a sufficient amount of vitamin cofactors to use, you are going to run into problems because your enzymes are not working for you, they are working against you.
Managing homocysteine levels through supplementation can help normalize the long term effects of genetic mutations affecting homocysteine metabolism. For example, If you have the C667T MTHFR mutation you will have about 25% higher homocysteine levels than people with a properly functioning MTHFR enzyme. You will benefit from adopting a specific supplementation strategy by ensuring your enzymes will always have access to the necessary vitamin cofactors. Often, medical professionals test homocysteine levels neglecting genetic mutations that are strongly influencing homocysteine levels like MTHFR mutations. We recommend to everyone they get checked to see if they have an MTHFR mutation. MTHFR mutations do not directly cause the problems of too much homocysteine but are the root of the problem. An MTHFR test should be given to anyone who is trying to get pregnant or suspects they may have hyperhomocysteinemia. The metabolically inefficient nature of MTHFR mutations leads to a buildup of homocysteine within the body.
Too much homocysteine leads to hyperhomocysteinemia. A condition increasing the likelihood of adverse events during pregnancy such as pre-eclampsia, placental abruption, neural tube defects (NTDs), Intrauterine growth retardation (IGR), recurrent pregnancy loss, and fetal death in utero. Hyperhomocysteinemia also contributes to the development of cardiovascular issues and mental illness.
At first glance, trying to understand homocysteine can be overwhelming. In the graphic below you can see many reactions taking place. We are going to be focusing primarily on reactions relating to homocysteine (located in the bottom right corner). You can see that methylfolate (5-MTHF) is the end result of the folate cycle (folate → 5-MTHF). The process of converting homocysteine to methionine is called methionine salvage and the converting homocysteine into cysteine is called transsulfuration. We will go over both the processes of methionine salvage and transsulfuration in detail.

The enzyme methionine synthase, along with its cofactor B12, catalyzes the reaction between 5-MTHF and homocysteine. This process produces methionine. If there is not enough B12 available in the system, or not enough 5-MTHF, methionine salvage will take much longer or will not happen at all. Not enough methionine synthase activity leads to less methionine salvage. Less methionine salvage leads to less methionine production and a buildup of homocysteine. When homocysteine begins building up, you become at risk for developing hyperhomocysteinemia that can lead to a variety of health related conditions.
Homocysteine And Methionine
Methionine is an essential amino acid, meaning it cannot be made by the body. The standard American diet contains high levels of methionine because meat contains high levels of methionine. Unless you are vegetarian, vegan, or have a deficiency in hydrochloric acid (HCL) production, you do not need to worry about being deficient in methionine. Methionine plays a role in the formation and maintenance of blood vessels, cartilage, and is an important building block of many proteins such as melatonin (helps you fall asleep) and carnitine (fat metabolism). Foods containing high levels of methionine include:
- Nuts
-
-
- Brazil nuts
- Cashew nuts
- Sesame seeds
- Flaxseeds
- Chia seeds
-
- Meats
-
-
- Beef
- Chicken
- Turkey
- Pork
-
- Seafood
-
-
- Tuna
- Salmon
- Halibut
- Snapper
- Shrimp
- Mussels
-
- Dairy
-
- Cheese
- Yogurt
- Milk
- Sour cream
Methionine is the precursor molecule to s-adenosyl methionine (SAM), the primary methyl donor. Low levels of SAM results in major methylation problems throughout the body. When SAM donates a methyl group it turns into s-adenosyl-l-homocysteine which is then made back into homocysteine.
Homocysteine And Cysteine
Cysteine is made by homocysteine through two metabolic reactions and is the process of transsulfuration. First homocysteine is made into cystathionine, through a reaction between homocysteine and cystathionine beta-synthase (CBS), an enzyme using vitamin B6 as a cofactor. Cystathionine goes through another reaction with another enzyme called cystathionine gamma-lyase (CGL) to produce cysteine. When cysteine reacts within the body is does so through oxidation, meaning it donates electrons to other molecules for the formation of new molecular bonds.
Cysteine is a highly reactive molecule participating in many reactions throughout the body. It contains a sulfur group allowing cysteine to form strong bonds with other molecules. Cysteine contributes in the formation of many functional proteins in the body. The most important of these proteins is glutathione. Glutathione is a powerful antioxidant made from cysteine, glutamate, and glycine. Life without glutathione would not be possible. It is vital for survival.
Take Aways
There are two potential pathways for homocysteine to go down. It can be made into methionine with the help of the MTR and BHMT enzymes or made into cysteine. Depending on the biochemical environment within your cells and within your body overall, one of the two pathways will be given priority over the other. Both pathways require different enzymes and different B vitamins that work together to give your body methionine, cysteine and/or glutathione to use.
There are essential B vitamins we need for our folate and homocysteine metabolism to run smoothly. The major pathway to make homocysteine into methionine requires B12 and the minor pathway requires betaine-homocysteine S-methyltransferase and TMG as a cofactor. Both enzymes responsible for the major and minor pathways transforming homocysteine into methionine require cofactors that are B vitamins (B12 and trimethylglycine) Homocysteine to cysteine requires vitamin B6. If at any time there is too much or too little B vitamins, this system can easily become unstable. Making sure you are getting the proper amount of B vitamins is important for keeping the folate and cysteine pathways functioning optimally.
Want more information on starting your supplements with the MTHFR gene mutation? Check out “How to Start your MTHFR Products”.
5 Common MTHFR Symptoms and How to Manage Your Gene Mutation
MTHFR Symptoms are brought on by MTHFR gene mutations and commonly underlie many health problems.
With a few simple changes and some professional advice, you may be able to alleviate some symptoms laid out in this article. MTHFR is often overlooked. New research is showing how important it really is. You may be shocked to find out how many symptoms are linked to MTHFR gene mutations.
MTHFR gene mutations may lead to less methylation in your body. Less methylation means that a variety of complex biochemical changes can change over time, leading to health problems. Since everyone is unique, (for example family history, lifestyle, diet etc..) symptoms arising from MTHFR mutations vary from person to person.
Fortunately, depending on how your MTHFR mutation is affecting you, you can see vast improvements in your health by treating MTHFR mutations and restoring methylation. Knowing you have a gene mutation is the first step to treating MTHFR symptoms associated.
5 Common MTHFR Symptoms Linked To MTHFR Gene Mutations
-
Cardiovascular Conditions
-
- MTHFR mutation may be adding fuel to the fire of cardiovascular problems. The following is a list of cardiovascular conditions where MTHFR combined with an elevated homocysteine could be playing a role:
- High blood pressure
- Stroke
- Heart attack
- Deep vein thrombosis
- MTHFR mutation may be adding fuel to the fire of cardiovascular problems. The following is a list of cardiovascular conditions where MTHFR combined with an elevated homocysteine could be playing a role:
-
-
Multiple Miscarriages and troubles falling pregnant.
- Pregnancy is one of the most beautiful and complicated biological processes on earth. Due to the complex nature of pregnancy, the most important nutrients required for the baby’s growth and development and good DNA from Mum and Dad is active folate.Ensuring your active folate production is in tip-top shape during preconception and pregnancy is critical for a healthy and successful pregnancy.
- If you are thinking about becoming pregnant and want to learn more about MTHFR, preconception, and pregnancy. Check out Carolyn’s free checklist “10 most important things you need to do before you become pregnant”
- Problems arising from MTHFR mutation during pregnancy include:
- Miscarriage
- Down syndrome
- Neural tube defects (Cleft lip/palate and spina bifida)
- Elevated blood pressure
- Anencephaly, encephalocele
- Pregnancy is one of the most beautiful and complicated biological processes on earth. Due to the complex nature of pregnancy, the most important nutrients required for the baby’s growth and development and good DNA from Mum and Dad is active folate.Ensuring your active folate production is in tip-top shape during preconception and pregnancy is critical for a healthy and successful pregnancy.
-
Oestrogen Dominance
- Methylation plays a crucial role in the production/regulation of hormones within the human body. In some cases, problems with methylation are the underlying factor behind Oestrogen dominance, which can produce the following symptoms:
- Fibrocystic ovaries and breasts
- Tenderness and swelling of breasts
- Mood swings
- Irregular menstruation cycle
- Food cravings
- Thyroid dysfunction
- Heavy periods
- Painful Periods
- Endometriosis (overgrowth of tissue lining the uterus, this is often painful)
- Fibroids
- Methylation plays a crucial role in the production/regulation of hormones within the human body. In some cases, problems with methylation are the underlying factor behind Oestrogen dominance, which can produce the following symptoms:
-
Mental Health Problems
- Mental health problems can be influenced by imbalances in hormones and neurotransmitters caused by a lack of methylation. Remember that SAMe is essential for our brain chemicals and your active folate helps you build SAMe levels. Receiving treatment for your faulty MTHFR gene may aid in improving/relieving the following symptoms:
- Depression
- Anxiety
- Bipolar
- Migraines
- ADD/ADHD
- Brain fog (difficulty concentrating)
- Mental health problems can be influenced by imbalances in hormones and neurotransmitters caused by a lack of methylation. Remember that SAMe is essential for our brain chemicals and your active folate helps you build SAMe levels. Receiving treatment for your faulty MTHFR gene may aid in improving/relieving the following symptoms:
-
General MTHFR Symptoms and Conditions Of MTHFR Gene Mutations Include:
- Chronic inflammation
- Chronic fatigue
- Dizziness
- Hives
- Allergies
- Eczema
- Asthma
- Heightened reaction to allergens
- Elevated liver enzymes
- Increased red blood cell folate
- Decreased white cell levels
- Decreased immune function
- High Homocysteine levels
- Low B12
Common Medications That Reduce Methylation
Common medications may be reducing your ability to methylate by lowering B12 or overall folate levels. If less B12 and/or folate is available to use in your body, symptoms may become worse. Medications that could be interfering with your B12 and folate levels include:
- Antacids
- Cholestyramine
- Nitrous oxide – your dentist will often use this. Its known as laughing gas.
- Methotrexate
- Niacin (at high doses) – because Niacin uses methyl groups in its metabolism
- Theophylline
- Cyclosporin
- Metformin
- Phenytoin
- Bactrim – an antibiotic
- Sulfasalazine
- Triamterene
- Trimethoprim
- Ethanol
- Oral Contraceptive Pill
- Antimalarials
Conditions of Hypomethylation (Under Methylation)
MTHFR mutations cause widespread undermethylation throughout the body. Undermethylation is responsible for an enormous amount of health conditions that prevent you from living life to its fullest. Below you can find a comprehensive list (organized categorically) of conditions associated with undermethylation:
General Physiological Malfunctions
- Allergic conditions
- Aging
- Alzheimer’s Disease
- Asthma
- Autoimmune Disease
- Cancer
- Chronic degenerative diseases
- Cardiovascular disease
- Chronic fatigue
- Diabetes
- Poor detoxification
- Fibromyalgia
- Headaches
- Joint pain, stiffness, and swelling
- Muscle pain
- Low neurotransmitter levels
- Obesity
- Increased pain
- Thyroid dysfunction
Mental Health
- ADD/ADHD
- Addictive behaviors
- Anorexia
- Anxiety
- Bipolar
- Bulimia
- Delusions
- Depression
- Autism
- Insomnia
- Obsessive-compulsive disorder (OCD)
- Oppositional defiant disorder (ODD)
- Phobias
- Psychosis
- Schizophrenia
Pregnancy/Development
- Spina bifida
- Anencephaly
- Encephalocele
- Iniencephaly
- Down syndrome
- Infertility
- Polycystic ovary syndrome
- Recurrent pregnancy loss/miscarriage
Improving Your Health With An MTHFR Mutation
If you want to take back control of your methylation cycle right away, supplementing is a great way to fight back against your MTHFR gene mutation. Care must be taken when supplementing to ensure symptoms do not worsen. To get started now, you can take a look at our “How to get started with MTHFR supplements” video below, or, download our guide on how to start supplementing MTHFR products.
P.S. Carolyn has also prepared a full overview of the MTHFR Gene Mutation and Management. Grab a good spot to listen and take some time to educate yourself with this Patient Webinar, exclusive to our audience. In this video, Carolyn gives you a thorough overview of MTHFR and symptoms related.
How the MTHFR gene mutation Affects fertility and pregnancy
One of the most common things I hear is the utter confusion many people find themselves in after they:
- Just discovered the MTHFR gene mutation and fell down an internet rabbit hole try to research and understand it all
- Were tested for it but received no explanation on its effects
- Were prescribed treatments that instead of helping made them feel worse
- Were told it may be one of the facets playing a role in their present health issues, but have no idea what it is
With the wealth of scientific (and at times confusing and complex) information found online surrounding MTHFR, it is no wonder many end up going around in circles and getting confused!
This is can be especially amplified in the case of those searching for clear and reliable information when it comes to the MTHFR gene mutation effects on fertility/preconception and pregnancy, as they are both very important topics where the health of both mother and child can rely on thoroughly understanding this vital information!
This article aims to plainly spell out what MTHFR actually is in the scope of preconception and pregnancy, both highlighting its general role in the body, and a vital role both before and during pregnancy.
So, What Is MTHFR
MTHFR stands for methylene-tetrahydrofolate reductase.
It is an enzyme in your body that converts the folate you eat (like in leafy greens and legumes) into the active form called 5-MTHF, or, 5-Methyltetrahydrofolate.
This means, that if you have a mutation in your MTHFR gene, the ability for your body to create healthy levels of active folate will be affected or decreased. This will result in less active folate available for your body to use in several very important processes.
This is where the link between MTHFR gene mutation, preconception and pregnancy arises. Several of the vital processes that we need active folate for are highly active during times of preconception and pregnancy and need to be working well to support a healthy pregnancy.
During preconception and pregnancy, you need good levels of active folate for:
- Creating healthy DNA for both you and your future child. This is the biggest reason why knowing your MTHFR gene result is so important. Active folate is directly involved in the synthesis of new DNA. And while we have a constant demand for the production of new and healthy DNA, you can imagine that demand for this hugely increases during pregnancy, when you are growing a new life! Issues with not enough healthy DNA available for both mother and growing child can result in issues with pregnancy, fetal growth, and general childhood development.
- Preventing levels of a substance in the body called homocysteine from climbing too high, which can be related to blood clots and increased risk of blot clot formation during pregnancy.
- To create molecules called ‘methyl groups’, which act as instruction manuals for your DNA and cells, telling them the correct way to ‘behave’, so they do not do anything unwanted (e.g. cause disease or dysfunction within the body). We need healthy levels of these methyl groups to methylate/instruct your DNA, and without it cells are uncontrolled and can start to cause problems.
- Formation of red blood cells, white blood cells, and platelets, which are all vital for both the health of the mother during pregnancy and also for the health of the child during pregnancy and after birth as they begin to rapidly grow and come into contact with bacteria and pathogens to strengthen their immune system.
As you can see, addressing and supporting your MTHFR genes during your preconception phase is the best way to healthily support both your body once you fall pregnant, and the growth and development of your new baby.
Knowing your MTHFR gene result and supporting your folate levels where needed is a key step in preconception, and both should not be undervalued!
Carolyn Ledowsky,
Founder of MTHFR Fertility
PS: If you are looking for a more in-depth explanation and management of MTHFR before pregnancy, be sure to check out our Flagship course “MTHFR and Preconception“. This 4 to 8 module course will guide you through the ins and outs of preparation your body will need to have a healthy and thriving pregnancy. Modules include what tests to expect, how to analyze the results and steps to take to prepare mentally, physically and emotionally for your pregnancy.”
How Does Folic Acid Supplementing or Fortifying Affect Your Body?
There is a lot of buzz around folates and folic acid.
Health professionals often find the terminology around folate and folic acid confusing. They use the words folic acid supplements when they mean folate, folinic acid or 5-methyltetrahydrofolate (active form). The mix up occurs because folate has become an umbrella term for many forms of folate. Each study may use a specific form of folate such as 5-methyltetrahydrofolate but by the time the study makes it to the media, for convenience, it’s being referred to as folate. This creates a game of broken telephone lines between the researcher and the public, leading to confusion.
There are many forms of folate. Fortunately, you only need to know about the forms of folate relevant to you. It is easy to get confused, so we are going to walk you through why each form of folate is important.
Is Folic Acid Equal To Folate?
No!
Folates are found naturally in food and folic acid is a man made synthetic form. The body prefers to use naturally occurring folates over the folic acid. Folic acid is a stable synthetic compound making it easy to fortify food with. Folic acid can be found in supplements and fortified foods (ex. Bread, baked goods, cereals or any other foods made with folate enriched wheat). We need less than 200 micrograms of folate per day! But, if you have an MTHFR mutation your body will often require more than 200 micrograms of folate per day.
Folic acid has no function in your body until it is made into dihydrofolate. Having too much folic acid in the system blocks the pathway for naturally occurring folates. Folic acid inhibits the DHFR enzyme from making natural folates into dihydrofolate.This is why folic acid supplements are usually a bad idea. Not until recently, due to advances in technology, 5-methyltetrahydrofolate and folinic acid became available for supplementation.
Supplementing with 5-methyltetrahydrofolate or folinic acid is better than folic acid. Depending on your unique situation a folate supplement may not be the best option for you or may require different supplementation strategies. A combination of 5-methyltetrahydrofolate and folinic acid at ratios specific to your condition may be recommended or some of you will do better on just active methyl folate. Working with a healthcare practitioner who is specialized in working with clients with MTHFR mutations is recommended because not getting the ratios right can lead to more health problems.
For more information on MTHFR and folic acid and folic acid supplements read our article “Why isn’t folic acid good for those with an MTHFR mutation? ” and for more information about supplementing folate read “A guide to folate supplementation”
https://www.mthfrsupport.com.au/folate-and-brain/
https://www.mthfrsupport.com.au/folic-acid-vs-5-mthf-treating-mthfr-deficiency/
https://www.mthfrsupport.com.au/top-20-folate-containing-foods/
https://www.mthfrsupport.com.au/folic-acid-vs-5-mthf-debate/
Why Is Folate Important?

Folate is widely studied because it plays many critical roles within the body. Folate plays a role in the following processes:
- Synthesis of DNA and RNA
- Use of S-adenosyl methionine (SAM) for the transfer of methyl groups
- Production and recycling of neurotransmitters
- Detoxification
- Creation of functional red blood cells (RBC), white blood cells (WBC) and blood platelets
A lack of folate can lead to harmful conditions, especially before or during pregnancy. Low folate levels are linked to:
- Neural tube defects
- Anencephaly
- Spina bifida
- Encephalocele
- Poor quality sperm
- Blood clotting issues leading to increased risk of thrombosis
- Autism
- Behavioral issues such as ADD/ADHD
- Increased sensitivity to allergens
- Low birth weight caused by
- Premature birth
- Slow prenatal growth rate
- Multiple miscarriages
Folinic acid (also known as calcium folinate or 5-formyltetrahydrofolate) is a cofactor in many reactions playing a role in:
- Purine synthesis
- Pyrimidine synthesis
- Amino acid conversion
- All are important for synthesis of DNA
5-methyltetrahydrofolate is the active form of folate in the body. Once made, it plays a role in the processes mentioned above but it also plays a vital role in the homocysteine cycle.
If 5-MTHF is out of balance you can start running into problems. Supplementing 5-methyltetrahydrofolate without professional guidance may lead to biochemical imbalances within your body. These imbalances can cause a variety of side effects including:
- irritability
- insomnia
- sore muscles
- achy joints
- acne
- rash
- severe anxiety
- palpitations
- nausea
- headaches
- migraines
- depression
- suicidal thoughts
What Are Natural Sources Of Folate?
Folate primarily comes from some of the healthiest foods you can eat. Dark green leafy greens. Here is a list of foods known to be high in folate:
- Spinach
- Kale
- Swiss Chard
- Collard greens
- Romaine lettuce
- Brussel sprouts
- Asparagus
- Broccoli
- Cauliflower
- Avocados
- Okra
Folate can also be found in some nuts, lentils, and beans. For a more detailed article about folate containing foods including folate-fortified foods, click here.
Overview Of The Folate Cycle
The diagram below shows a simplified process of the necessary steps to turn folate to its active form 5-MTHF within the body.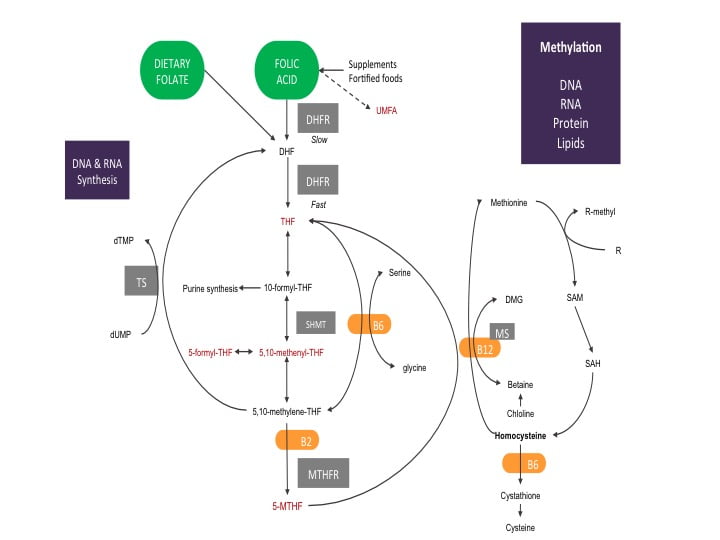
Starting with dietary folate, you can see it is ready to jump right into the folate cycle. The folate you eat is quickly made into dihydrofolate (DHF). DHF is given electrons by the enzyme dihydrofolate reductase (DHFR) using one molecule of nicotinamide adenine dinucleotide phosphate (NADPH, a common electron donor within the body and participates in many biochemical reactions) to make tetrahydrofolate (THF).
The diagram shows DHFR is quicker at making THF from dietary folate compared to folic acid. This is because the DHFR enzyme has more attraction to dietary folate and only needs to help with one reaction. Folic acid requires two reactions with the DHFR enzyme to become THF. The first reaction of folic acid is slow. The slow reaction process uses up available DHFR enzymes, blocking them from using natural folates.
The more folic acid present within the system, the slower folate metabolism becomes. If the system becomes saturated with folic acid, some research suggests DHFR enzymes can become completely blocked. A building up of folic acid begins, causing the folate cycle to become out of balance. Anytime an important biochemical process within the body becomes out of sync with its natural cycles there are negative health consequences. If you are trying to become pregnant or are experiencing treatment-resistant health issues, bringing the folate cycle back into its natural cycle is important for ensuring a healthy pregnancy and restoring the body’s methylation system. For a more in-depth article on the folate cycle read our article “The Folate Cycle In Detail”
Changing Your Relationship To Folate
There are major differences between types of folates. Keeping this in mind when choosing what to eat throughout the day. Avoiding foods fortified with folic acid is a good place to start. Unfortunately, folic acid fortification is mandatory in 53 countries around the world. Food such as bread, baked good, cereals are made with enriched wheat and are going to be fortified with folic acid. Making the choice to substitute folate fortified food for food containing natural folates can make a big difference in maintaining a healthy and functioning folate cycle.
Make sure you are including more dark leafy greens in your diet. Refer to our folate containing foods list to get a general sense of what contains folate when planning out your meals. MTHFR mutations decrease your ability to process folate. If you have an MTHFR mutation, avoiding foods fortified with folic acid and getting your natural folates into your diet should be a priority for you. If your health conditions persist after improving your dietary folate intake, you should speak with your healthcare practitioner about supplementing with a combination of active folates. Your situation may be more complex, requiring a specific supplementation strategy to restore a natural and healthy folate cycle.
You can join Carolyn for her next FREE webinar on “What is MTHFR?” for more information about MTHFR and folate. Watch our Events page for updates!
Take home message
The key message here is that we should avoid folic acid where possible, particularly in supplement form and fortified foods, particularly if you have an MTHFR gene mutation. You should be getting as much folate as you can from your diet in the way of leafy green vegetables, and if you have health conditions speak to your practitioner about supplementing with various combinations of active folates ie: folinic acid or 5-MTHF.
References
[1] Aarabi, M., San Gabriel, M. C., Chan, D., Behan, N. A., Caron, M., Pastinen, T., … & Trasler, J. (2015). High-dose folic acid supplementation alters the human sperm methylome and is influenced by the MTHFR C677T polymorphism. Human molecular genetics, 24(22), 6301-6313.
[2]Hekmatdoost, A., Vahid, F., Yari, Z., Sadeghi, M., Eini-Zinab, H., Lakpour, N., & Arefi, S. (2015). Methyltetrahydrofolate vs Folic Acid Supplementation in Idiopathic Recurrent Miscarriage with Respect to Methylenetetrahydrofolate Reductase C677T and A1298C Polymorphisms: A Randomized Controlled Trial. PloS one, 10(12), e0143569.
[3] https://www.cdc.gov/ncbddd/folicacid/recommendations.html
[4] https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/
[5] Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. (1998). Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. National Academies Press (US).
[6] Jacques, P. F., Bostom, A. G., Williams, R. R., Ellison, R. C., Eckfeldt, J. H., Rosenberg, I. H., … & Rozen, R. (1996). Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation, 93(1), 7-9.
Taking Folic Acid When You Have An MTHFR Mutation | Why Is It Not Recommended
So you have one of the common MTHFR mutations and are not sure how to start supplementing. You went online to find out what to take and read that taking folic acid is a good idea. We are here to tell you it’s NOT. Check out our article “Using Folic Acid Supplements In Fertility Treatments” for an overview. Once you are up to speed on folic acid, take a look at where it enters the folate cycle in the graphic below.
You can see folic acid entering the folate cycle right at the beginning. It’s the first molecule setting the folate cycle in motion, but it’s not the best molecule to start the process of folate metabolism. This type requires an extra metabolic step, that is slow and inefficient, before entering the folate cycle where naturally occurring folates do.
Folic Acid Vs. Natural Folates
Folic acid is a synthetic form of folate (vitamin B9) that are made to be more chemically stable. It is made to be more stable than naturally occurring folates because they are not broken down during the manufacturing process as much. Companies can get folic acid to the shelf a lot easier than they can get naturally occurring folates. The companies want to make as much money as possible so they market and sell synthetic product over naturally occurring folates. Extracting, processing and storing naturally occurring folates is difficult and expensive. Many of the folates at the beginning of the manufacturing process end up breaking down, rendering them useless. Folic acid was the first form of folate available but in recent years the active forms are now able to be put in supplements.
Dihydrofolate Reductase (DHFR)
The dihydrofolate reductase (DHFR) enzyme breaks down folic acid and natural folates into tetrahydrofolate (THF). Taking folic acid can reduce your ability to metabolize naturally occurring folates you are getting through your diet. The graphic shows DHFR acting slowly on folic acid and fast on dietary folates. This is because it is made to be a more stable molecule compared to non-synthetic forms of folate. The chemical stability and slight change in shape of folic acid compared to natural folates, make it difficult for DHFR to use/metabolize. The reaction takes much longer to happen because the chemical structure of folic acid is resistant to metabolism by DHFR.
There are a limited number of DHFR enzymes available for metabolizing folic acid and dietary folates. When most of the folates in your diet are coming from synthetic supplementation or from foods fortified with folic acid, the DHFR enzymes are busy trying to metabolize folic acid instead of naturally occurring folates.
The DHFR enzyme itself can only metabolize 200 mcg of folic acid per day. If you are getting over 200 mcg of folic acid through supplementing and/or from fortified foods, the DHFR enzyme is going to be inhibited. This causes a build of folic acid and folates at the beginning of the folate cycle within the body. The build-up of folic acid is called un-metabolized serum folic acid or UMFA for short.
For more information about UMFA read our article Folic Acid: What Happens When You Have Too Much?
Take Aways and MTHFR
Too much folic acid in your diet from fortified foods, or taking folic acid supplements, is going to reduce your ability to metabolize folates you are getting from your diet. It is best to stay away from foods fortified with folic acid when you can and eat more food with high levels of naturally occurring folates. Since folic acid will build up in the folate cycle, there will be less folate making it through the final stage of the cycle which requires the MTHFR enzyme. If you have an MTHFR mutation, you know your MTHFR enzyme has a 20-70% loss in function when converting 5,10-methylene-THF to 5-MTHF. Including folic acid in your diet or supplement makes the entire folate cycle even less efficient! You need to avoid taking folic acid and adopt a diet full of natural folates, especially if you have an MTHFR mutation! Here you can find out the Top 20 Folate Containing Foods.
For some people, a consistent and high folate diet may be difficult. If you are worried about not getting enough folates, there are better ways to get folates through supplementation than through a synthetic method, check out our Guide To Folate Supplementation.
If you are preparing your body for a healthy and thriving pregnancy, we have a free 10-day email course we recommend to get you started on the right path. Sign up for free today and get started on the best path for you and a thriving pregnancy.
Folate Supplementation Guide
Folate Supplementation Guide
You are looking to get pregnant and want to do all you can to ensure you have a happy and healthy baby. When done correctly, folate supplementation can make a huge impact on your child’s health during pregnancy!
The word folate is an umbrella term, describing multiple forms of folate. Using the term folate keeps things simple, but when you dig deeper beyond the umbrella you discover folate has many faces.
There are three forms of folates you should be aware of when beginning to think about folate supplementation.
- Folic Acid
- Folinic Acid
- 5-MTHF
This article will explain the basics of these 3 forms of folate for supplementation purposes. You will learn about which supplements help move the folate cycle along, what advantages each supplement has over others, what folate supplements to stay away from, and when to begin taking folate supplements.
When To Supplement with Folate
Folate supplementation should start long before pregnancy begins. Ensuring proper folate levels during preconception preparation is something everyone hoping to become pregnant should do. It takes time for the body to get the folate cycle back up and running efficiently (at least a couple weeks). If there is a problem such as a MTHFR mutation or other problems affecting the folate cycle, it may take longer to normalize. Folate supplementation should begin during preconception and continue throughout the entire pregnancy.
You want to prevent your body from having low folate levels long before you reach the periconceptional period of your pregnancy. During the first 3-4 weeks of pregnancy (periconceptional period), low folate levels play a role in the development of neural tube defects (NTD’s) and many other pregnancy complications, which are listed in our article “What is folic acid?”.
Folic Acid (Not Recommended)
The daily recommended intake of folic acid is 400 mcg. Common multivitamins will give you around 400 mcg of folic acid. If you are taking a multivitamin for folic acid, taking another folic acid supplement is going to be overkill. You will be getting too much folic acid. You and your child will be at risk for developing neurological disorders because folic acid at high levels is toxic to the human nervous system.
The one advantage folic acid has over other supplementation option is that it’s inexpensive. Folic acid is a synthetic compound that can be easily stored, transported and has a long shelf life. Other than being less expensive than other forms of folate supplementation, folic acid comes up short for the following three reasons:
- Folic acid is a synthetic compound that has NO physiological function until converted to dihydrofolate by dihydrofolate reductase (DHFR)
- The DHFR enzyme breaks down folic acid much slower than naturally occurring folates which causes a build up of folic acid.
- Folic acid has a stronger attraction to the folate receptors, blocking them from pulling natural folates into the cell for metabolic processes (our natural folates like leafy greens are essential for our folate levels)
When you take folic acid your body is not going to make use of the best folates available. This is due to the compounding of your cells folate receptors having a preference for folic acid and your enzymes being slower to react with folic acid. The DHFR enzyme has trouble metabolizing folic acid and on top of that, the DHFR enzyme is susceptible to mutations that reduce its functionality. If you have or think you have an MTHFR mutation, folic acid supplementation can create even more problems for your folate cycle. You can read more about “Why Folic Acid Is Not Good If You Have A MTHFR Mutation”
Folinic Acid (Recommended)
You can avoid the pitfalls of folic acid supplementation and/or a harmful DHFR mutation, by supplementing with folinic acid. Folinic acid enters the folate cycle in a better position than folic acid. Folinic acid or 5-formyltetrahydrofolate, enters directly into the middle of the folate cycle. It can be made into any of the possible products of the folate pathway, making it ideal for people who have DHFR mutations, or people who are exposing themselves to high levels of folic acid by consuming folic acid fortified foods.
Folinic acid directly contributes to physiological processes, unlike folic acid which has no direct physiological function. Folinic acid aids the synthesis of DNA by acting as a cofactor in the metabolic reactions responsible for creating purine and pyrimidine (two of the four building blocks of DNA).
There are three main points regarding folinic acid you should take away with you:
- Folinic acid can be made into everything folic acid or natural folates can be made into
- Folinic acid has direct physiological functions that help create the building blocks of DNA
- Folinic acid bypasses the DHFR enzyme in the folate cycle, making it an ideal supplementation strategy if you have a DHFR mutation that reduces your ability to metabolize folates and folic acid
5-MTHF or Active Folate (Recommended)
If you know you have an MTHFR mutation, 5-MTHF is going to be the most important supplement for you to take. 5-MTHF is the active form of folate that allows the body to recycle its methyl donors (the molecules responsible for turning off and on physiological processes within the human body by donating methyl groups).
The folate cycle is designed to produce 5-MTHF. 5-MTHF is the end product of the folate cycle and its production is directly affected by the MTHFR enzyme. Having an MTHFR gene mutation reduces your body’s natural ability to produce its active form of folate. When you read about the problems caused by a lack of folate, it’s due to a lack of the bodies active form of folate, 5-MTHF. Below is a quick recap of the problems linked to low folate status:
- Neural tube defects
- Lower sperm quality
- Blood clotting disorders (thrombophilia)
- Autism
- ADD/ADHD
- Allergies
- Low birth rate
An eye should be kept on your folate status if you are trying to become pregnant. During pregnancy, there are high volumes of DNA being produced every day. The child is growing rapidly and has the highest risk of being negatively affected by low folate levels.
The body is a highly complex system, and when you change your diet, your environment, or start supplementing to improve your folate status, it takes a couple months for your body to adjust to healthy folate levels.
Take Aways
Supplementing folates properly, by taking the right supplements (Folinic acid and 5-MTHF) and avoiding getting too much folic acid, will ensure you are managing your MTHFR mutation in a safe way. Leaving your MTHFR mutation as is, without taking steps to reduce its negative impact, is not good for you or pregnancy. Taking the necessary steps to ensure proper methylation throughout the body by maintaining sufficient folate levels through supplementation, if needed, will greatly reduce the risk of developmental health defects for your child.
Folic Acid: What Happens When You Have Too Much?
What The UMFA?
UMFA stands for unmetabolized folic acid. If left unchecked, UMFA can cause a variety of health problems, but we will go over those later! The majority of folate found within the blood is 5-methyltetrahydrofolate (MTHF), which is not going to harm you; unlike UMFA! There is no need to worry about UMFA if you are getting all your folate from natural sources. However, if you are supplementing or eating a lot of foods fortified with folic acid, you may have a considerable amount of UMFA circulating through your blood!
What Are The Health Consequences Of Too Much Folic Acid And The Build Up Of UMFA?
Your body produces UMFA when it is unable to metabolize folic acid. The rate of folate metabolism is different for everyone, but generally, the body breaks down folic acid slower than folate from natural sources (4). Only people who are supplementing with or consuming folic acid fortified foods are at risk for having UMFA present in their blood. UMFA can cause a variety of problems within the body including (9,11):
- Impairment of the immune system
- Cancer grows more easily
- Lowers iron
- Slows cognition
- Impairs memory
Why UMFA Builds Up In The Body
Now that you are aware of the health consequences of UMFA, it’s important to know how and why UMFA begins to build up. The body metabolizes and moves folate around the body through several pathways (1,10):
- Tissue uptake and storage
- Secretion into bile
- Reabsorption
- Hepatic metabolism
- Renal excretion
If any of the listed folate pathways become saturated, the body will begin to build up UMFA within blood serum. Remember that the body has a harder time metabolizing synthetic folic acid compared to naturally occurring folates. See our article on “How Does Folic Acid Supplementing or Fortifying Affect Your Body?” for more information!
The majority of the burden to metabolize folic acid falls onto the liver; where the bulk of the DHFR enzyme (the enzyme responsible for metabolizing folates) is made. The liver has limited capacity to reduce folic acid into folate due to natural biological limitations. If you are supplementing >200ug of folic acid, it will lead to a temporary jump in unmetabolized folic acid in your blood serum (3,6).
3 Steps You Can Take To Reduce UMFA
There are three main ways to reduce folic acid and prevent the build-up of UMFA within your blood:
- Take folinic acid or 5-MTHF (For more information read our article A Guide to Folate Supplementation”)
- Avoid foods that are fortified with folic acid (fortified grain products)
- Eat more food that has naturally occurring folates (See our article on the top 20 folate containing foods https://www.mthfrsupport.com.au/top-20-folate-containing-foods/)
Take Aways
UMFA is only a problem if you are consuming folic acid on a regular basis. Eating some fortified foods every now and then is not going to kill you, but if you are having trouble becoming pregnant you need every advantage you can get. Reducing consumption of synthetic, and metabolically demanding, folic acid decreases the amount of UMFA found in blood serum — giving you a fertility advantage! 
References
- Aiso, K., Nagasue, M., Nozaki, T., Shimoda, M., & Kokue, E. (2001). Comparison of dihydrofolate reductase activities for folic acid in pigs and rats using in vivo and in vitro evaluation techniques. Journal of nutritional science and vitaminology, 47(2), 96-101. (4)
- Bailey, R. L., Mills, J. L., Yetley, E. A., Gahche, J. J., Pfeiffer, C. M., Dwyer, J. T., … & Picciano, M. F. (2010). Unmetabolized serum folic acid and its relation to folic acid intake from diet and supplements in a nationally representative sample of adults aged≥ 60 y in the United States. The American journal of clinical nutrition, 92(2), 383-389.
- Kelly, P., McPartlin, J., Goggins, M., Weir, D. G., & Scott, J. M. (1997). Unmetabolized folic acid in serum: acute studies in subjects consuming fortified food and supplements. The American journal of clinical nutrition, 65(6), 1790-1795.
- Mudryj, A. N., de Groh, M., Aukema, H. M., & Yu, N. (2016). Folate intakes from diet and supplements may place certain Canadians at risk for folic acid toxicity. British Journal of Nutrition, 116(7), 1236-1245.
- Obeid, R., Kirsch, S. H., Dilmann, S., Klein, C., Eckert, R., Geisel, J., & Herrmann, W. (2016). Folic acid causes higher prevalence of detectable unmetabolized folic acid in serum than B-complex: a randomized trial. European journal of nutrition, 55(3), 1021-1028.
- Patanwala, I., King, M. J., Barrett, D. A., Rose, J., Jackson, R., Hudson, M., … & Jones, D. E. (2014). Folic acid handling by the human gut: implications for food fortification and supplementation. The American journal of clinical nutrition, 100(2), 593-599.
- Pfeiffer, C. M., Sternberg, M. R., Fazili, Z., Lacher, D. A., Zhang, M., Johnson, C. L., … & Berry, R. J. (2015). Folate status and concentrations of serum folate forms in the US population: National Health and Nutrition Examination Survey 2011–2. British Journal of Nutrition, 113(12), 1965-1977.
- Pfeiffer, C. M., Sternberg, M. R., Fazili, Z., Yetley, E. A., Lacher, D. A., Bailey, R. L., & Johnson, C. L. (2015). Unmetabolized folic acid is detected in nearly all serum samples from US children, adolescents, and adults. The Journal of nutrition, jn-114.
- Plumptre, L., Masih, S. P., Ly, A., Aufreiter, S., Sohn, K. J., Croxford, R., … & Kim, Y. I. (2015). High concentrations of folate and unmetabolized folic acid in a cohort of pregnant Canadian women and umbilical cord blood. The American journal of clinical nutrition, 102(4), 848-857.
- Russell, R. M., Rosenberg, I. H., Wilson, P. D., Iber, F. L., Oaks, E. B., Giovetti, A. C., … & Press, A. W. (1983). Increased urinary excretion and prolonged turnover time of folic acid during ethanol ingestion. The American journal of clinical nutrition, 38(1), 64-70. (5)
- Sawaengsri, H., Wang, J., Reginaldo, C., Steluti, J., Wu, D., Meydani, S. N., … & Paul, L. (2016). High folic acid intake reduces natural killer cell cytotoxicity in aged mice. The Journal of nutritional biochemistry, 30, 102-107.