Importance of vitamin B12 and MTHFR
Importance of Vitamin B12 and MTHFR
Many of us are living with MTHFR mutations and are unaware of our MTHFR status, and a small number of us are taking advantage of knowing the MTHFR genes we have. If you do not know what MTHFR is, or would like to learn more about MTHFR, read our introduction to MTHFR here. Certain MTHFR mutations, such as MTHFR C667T or MTHFR A129C, contribute to many health and fertility problems. This article will discuss how MTHFR mutations negatively impact the methylation pathway and vitamin B12 levels, and also offer advice on what you can do to have better health outcomes living with an MTHFR mutation.
What’s the connection between MTHFR and vitamin B12
Folate and vitamin B12 are the most important cofactors working with enzymes in the methylation cycle. The methylation cycles begins with folate. Folate is converted into its active form (5-methyltetrahydrofolate) through the folate cycle, and MTHFR is directly responsible for the creation of folates active form. MTHFR deficiencies lead to a shortage of the active form of folate within the body and negatively impacts the conversion of homocysteine to methionine. Low active folate levels can lead to a build of of homocysteine and lack of methionine in the body and can lead to several health problems (more on high homocysteine levels can be found here).
High homocysteine levels can also be caused by low vitamin B12 levels because vitamin B12 is the cofactor needed by the enzyme responsible for converting homocysteine to methionine. Without adequate vitamin B12 levels a build of homocysteine begins occuring, and can be made even worse in combination with harmful MTHFR mutations. If you are curious to find out if you have MTHFR, we have different MTHFR testing kits available.
Australia – https://mthfrsupport.com.au/product-category/test-kits/
International – https://mthfr-fertility.myshopify.com/collections/lab-tests
What you need to know about vitamin B12 levels and MTHFR mutations
People living with MTHFR mutations commonly have issues with vitamin B12. They often show the symptoms of vitamin B12 deficiency, but when they get tested their vitamin B12 levels are normal. A healthy vitamin B12 level is between 500-800 µmol. You may be asking, why would a person suffer from the symptoms of vitamin B12 deficiency if they have healthy vitamin B12 levels?
The answer involves MTHFR mutations. MTHFR mutations impact the bodies ability to use vitamin B12. This happens because certain MTHFR mutations (C667T and A129C) lower the amount of active folate being produced in the body, and the use of vitamin B12 requires the active form of folate. Vitamin B12 deficiency can be occurring while vitamin B12 levels are at levels not typically associated with deficiency due to the presence of deleterious MTHFR mutations. If you are one of the unlucky people with symptoms of vitamin B12 deficiency and vitamin B12 levels within the normal range, the best things you can do would be to increase your folate intake through supplementation and eating more folate containing foods.
We sell a variety of supplements for folate and Vitamin B deficiencies that can be found here, a guide to folate supplementation can be found here, and you can learn more about what foods are high in folate here.
Symptoms of vitamin B12 deficiency
MTHFR mutations and vitamin B12 deficiencies commonly work together to produce elevated homocysteine levels which can damage the nervous system, leading to cognitive impairment, and increased risk of Alzheimer’s Disease and dementia. Elevated homocysteine levels are also associated with increased risk for cardiovascular issues such as strokes and heart attacks. Symptoms of vitamin B12 deficiency include: fatigue, irritability, anxiety, low energy, depression, forgetfulness, constipation, new food sensitivities, hot and cold flashes, sore muscles, pale skin, numbness, tingling, and dermatitis.
How to overcome B12 issues
There comes a time when it’s time to take health matters into your own hands. What’s the best way to overcome vitamin B12 deficiency and elevated homocysteine levels? There a a few for of vitamin B12 supplements available on the market. We will go over each form of vitamin B12 and tell you how to determine if it’s the right one for you.
Cyanocobalamin
Cyanocobalamin is a form of vitamin B12 derived from cyanide poison, and for this reason should be avoided. Long term supplementation with cyanocobalamin can have systemic toxic effects on the body amd even lead to kidney failure! Another downside of supplementing with cyanocobalamin is that it’s a form of vitamin B12 that requires an extra chemical reaction to occur within the liver until it becomes something your body can use. Making it more difficult to breakdown for those living with MTHFR mutations.
Hydroxocobalamin
Hydroxocobalamin is more bioavailable than cyanocobalamin and does not require extra reactions occuring in the liver before the body can use it. For this reason, hydroxocobalamin is recommended for people with MTHFR mutations, known methylation issues, and is the best supplement for anyone living with low blood pressure. Hydroxocobalamin should be taken as a preventative measure against complications that arise from vitamin b12 deficiencies, high homocysteine levels, and methylation issues.
Methylcobalamin
Methylcobalamin is another form of vitamin B12 that does not require extra processing in the liver, making it a great alternative to taking cyanocobalamin. Methylcobalamin is often taken to improve cardiovascular problems, cognitive impairments, behavioral issues associated with autism, and circadian rhythm disturbances.
Adenosylcobalamin
Adenosylcobalamin is great to take alongside methylcobalamin to obtain the full spectrum of benefits that can be derived from vitamin B12 supplementation. Adenosylcobalamin is the form of vitamin B12 associated most closely with improving energy levels in people who are deficient in vitamin B12.
Here are your best options for solving your B12 issues
Vitamin B12 deficiencies are fairly common, and now you have learned more about how to fix vitamin B12 deficiencies. If you are living with an MTHFR mutation, are vegetarian/vegan, or are susceptible to vitamin B12 deficiency for other reasons, you now have more tools in your tool box for understanding and dealing with vitamin B12 deficiencies. If you have troubles solving your vitamin B12 deficiency on your own, the next best step is to take what you have learned/tried to your doctor and work with them to solve your health issues associated with vitamin B12 deficiency.
However , before you go and see your doctor, it’s important to start supplementing with vitamin B12 and other B vitamins right away if you suspect you are deficient.
If you are looking for a more in-depth and personalized approach to MTHFR, vitamin B12 deficiency, or fertility issues than check out our patient resource center.
Using Folic Acid Supplements in Fertility Treatments
In my current series of preconception webinars, I am not surprised by the number of women joining me who have had multiple miscarriages or who are currently having IVF (often unsuccessfully). They didn’t know they had the MTHFR gene and even after multiple pregnancy losses, still were not checked for the gene. Eventually, they sought the answer themselves.
Folic Acid Supplements in Fertility Treatments
Folate is critical for DNA methylation and cell division. As a result, it’s also important for the proper development of ova, or egg cells, that can successfully implant in the uterus.
But when we say folate what do we mean?
Well, think of ‘folate’ as an umbrella term. Under that umbrella, there is folic acid (the synthetic man made form), folinic acid which is an important cofactor for healthy DNA creation, and our active folate, called5-MTHF (methyltetrahydrofolate).
In IVF and fertility issues, doctors and specialists alike are still recommending a high dose 5mg folic acid supplement to remedy a MTHFR mutation.
We also have mandatory folic acid supplementation in all our commercial bread and in many of our breakfast cereals, juices, protein bars, shakes, and energy drinks. So researchers are now looking into what these high doses are doing to us.
You all know that those with the MTHFR gene mutation are counseled into avoiding folic acid. The reason is that folic acid has the potential to build up and inhibit our DHFR enzyme, which is crucial in our folate pathway. Some research has also shown that unmetabolized folic acid builds up and affects our immune system negatively too.
But now we know more! Some really interesting studies on folic acid were released in the latter half of 2015 that specifically focused on folic acid. These studies looked at the 5mg dose of folic acid to see how it affected fertility. Now, this is interesting to me, because many of our patients in the clinic have been prescribed 5mg of folic acid in response to their doctor seeing a MTHFR C677T homozygous result, or to prepare their body before they start IVF cycles.
So let’s take a closer look:
We know that fertility is decreasing world wide and we have to ask ourselves why. Sure we have a more toxic environment than ever before; yes we are more stressed than previous generations and have worse diets and fewer nutrients in our soil. But a recent study completed in the Human Molecular Genetic Journal at the end of 2015 showed some interesting information. They looked at DNA methylation of the sperm when they gave folic acid. The researchers trialed 5mg of folic acid in infertile men and acknowledged that serum folate concentrations increased significantly after 6 months of folic acid supplementation. They also noticed a slight, but non-significant increase in sperm numbers, but the surprising thing is that they found a ‘significant loss of methylation across the sperm epigenome’, and more so if you were homozygous for the MTHFR C677T mutation.
What is more alarming is that the researchers suggested that this loss of methylation in sperm DNA might be transmitted to the offspring. So what they are saying is that folic acid at high doses not only decreases the fertility of men by negatively affecting their DNA, but also that these effects may be passed onto the child.
Is this significant? Could this be adding to the infertility effect we are seeing in men?
In the Clinical Journal of Nutrition in 2015, Karen Christensen found that high folic acid consumption reduces MTHFR protein and activity levels, creating a pseudo MTHFR deficiency in mice.This deficiency affects liver cells ability to metabolize fat and affects cell membrane integrity. That’s why we often see elevated cholesterol levels in people with MTHFR deficiency and issues with egg integrity in women undergoing IVF.
Another study looking at folic acid supplementation in people with the MTHFR gene going into IVF also revealed some interesting data. This Swedish study found that the higher the folic acid intake, the higher the plasma folate. Overall, the conclusion was that ‘high folic acid intake did not seem to assist infertile women to achieve pregnancy after fertility treatment’.
It’s the same result as above for the men.
So we seem to be getting more of the same. Yes, folic acid is going to increase serum folate levels, that much makes sense. However, it does not help DNA methylation, that’s why it’s not helping fertility.
Methylation of DNA is what controls our fertility. Just because we’ve always done something doesn’t make it right. There should be a worldwide review of folic acid supplementation in fertility treatment. There is enough research emerging to have a review of existing protocols.
Find out more about the study.

Founder MTHFR Support Australia
How Does Folic Acid Supplementing or Fortifying Affect Your Body?
There is a lot of buzz around folates and folic acid.
Health professionals often find the terminology around folate and folic acid confusing. They use the words folic acid supplements when they mean folate, folinic acid or 5-methyltetrahydrofolate (active form). The mix up occurs because folate has become an umbrella term for many forms of folate. Each study may use a specific form of folate such as 5-methyltetrahydrofolate but by the time the study makes it to the media, for convenience, it’s being referred to as folate. This creates a game of broken telephone lines between the researcher and the public, leading to confusion.
There are many forms of folate. Fortunately, you only need to know about the forms of folate relevant to you. It is easy to get confused, so we are going to walk you through why each form of folate is important.
Is Folic Acid Equal To Folate?
No!
Folates are found naturally in food and folic acid is a man made synthetic form. The body prefers to use naturally occurring folates over the folic acid. Folic acid is a stable synthetic compound making it easy to fortify food with. Folic acid can be found in supplements and fortified foods (ex. Bread, baked goods, cereals or any other foods made with folate enriched wheat). We need less than 200 micrograms of folate per day! But, if you have an MTHFR mutation your body will often require more than 200 micrograms of folate per day.
Folic acid has no function in your body until it is made into dihydrofolate. Having too much folic acid in the system blocks the pathway for naturally occurring folates. Folic acid inhibits the DHFR enzyme from making natural folates into dihydrofolate.This is why folic acid supplements are usually a bad idea. Not until recently, due to advances in technology, 5-methyltetrahydrofolate and folinic acid became available for supplementation.
Supplementing with 5-methyltetrahydrofolate or folinic acid is better than folic acid. Depending on your unique situation a folate supplement may not be the best option for you or may require different supplementation strategies. A combination of 5-methyltetrahydrofolate and folinic acid at ratios specific to your condition may be recommended or some of you will do better on just active methyl folate. Working with a healthcare practitioner who is specialized in working with clients with MTHFR mutations is recommended because not getting the ratios right can lead to more health problems.
For more information on MTHFR and folic acid and folic acid supplements read our article “Why isn’t folic acid good for those with an MTHFR mutation? ” and for more information about supplementing folate read “A guide to folate supplementation”
https://www.mthfrsupport.com.au/folate-and-brain/
https://www.mthfrsupport.com.au/folic-acid-vs-5-mthf-treating-mthfr-deficiency/
https://www.mthfrsupport.com.au/top-20-folate-containing-foods/
https://www.mthfrsupport.com.au/folic-acid-vs-5-mthf-debate/
Why Is Folate Important?

Folate is widely studied because it plays many critical roles within the body. Folate plays a role in the following processes:
- Synthesis of DNA and RNA
- Use of S-adenosyl methionine (SAM) for the transfer of methyl groups
- Production and recycling of neurotransmitters
- Detoxification
- Creation of functional red blood cells (RBC), white blood cells (WBC) and blood platelets
A lack of folate can lead to harmful conditions, especially before or during pregnancy. Low folate levels are linked to:
- Neural tube defects
- Anencephaly
- Spina bifida
- Encephalocele
- Poor quality sperm
- Blood clotting issues leading to increased risk of thrombosis
- Autism
- Behavioral issues such as ADD/ADHD
- Increased sensitivity to allergens
- Low birth weight caused by
- Premature birth
- Slow prenatal growth rate
- Multiple miscarriages
Folinic acid (also known as calcium folinate or 5-formyltetrahydrofolate) is a cofactor in many reactions playing a role in:
- Purine synthesis
- Pyrimidine synthesis
- Amino acid conversion
- All are important for synthesis of DNA
5-methyltetrahydrofolate is the active form of folate in the body. Once made, it plays a role in the processes mentioned above but it also plays a vital role in the homocysteine cycle.
If 5-MTHF is out of balance you can start running into problems. Supplementing 5-methyltetrahydrofolate without professional guidance may lead to biochemical imbalances within your body. These imbalances can cause a variety of side effects including:
- irritability
- insomnia
- sore muscles
- achy joints
- acne
- rash
- severe anxiety
- palpitations
- nausea
- headaches
- migraines
- depression
- suicidal thoughts
What Are Natural Sources Of Folate?
Folate primarily comes from some of the healthiest foods you can eat. Dark green leafy greens. Here is a list of foods known to be high in folate:
- Spinach
- Kale
- Swiss Chard
- Collard greens
- Romaine lettuce
- Brussel sprouts
- Asparagus
- Broccoli
- Cauliflower
- Avocados
- Okra
Folate can also be found in some nuts, lentils, and beans. For a more detailed article about folate containing foods including folate-fortified foods, click here.
Overview Of The Folate Cycle
The diagram below shows a simplified process of the necessary steps to turn folate to its active form 5-MTHF within the body.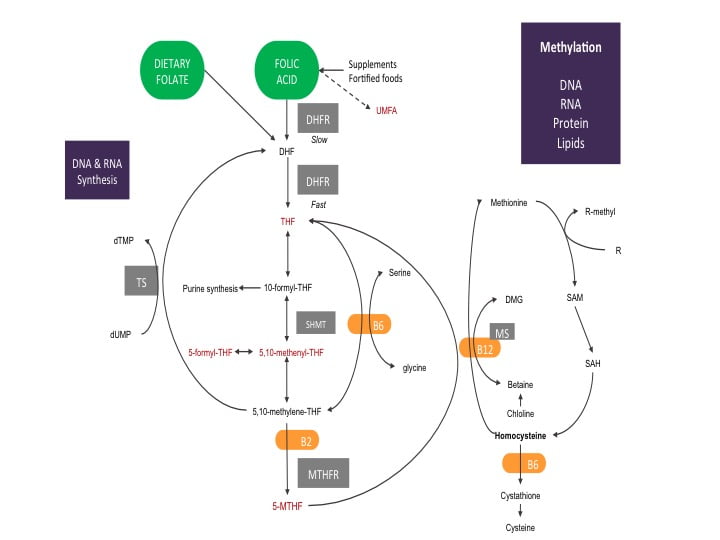
Starting with dietary folate, you can see it is ready to jump right into the folate cycle. The folate you eat is quickly made into dihydrofolate (DHF). DHF is given electrons by the enzyme dihydrofolate reductase (DHFR) using one molecule of nicotinamide adenine dinucleotide phosphate (NADPH, a common electron donor within the body and participates in many biochemical reactions) to make tetrahydrofolate (THF).
The diagram shows DHFR is quicker at making THF from dietary folate compared to folic acid. This is because the DHFR enzyme has more attraction to dietary folate and only needs to help with one reaction. Folic acid requires two reactions with the DHFR enzyme to become THF. The first reaction of folic acid is slow. The slow reaction process uses up available DHFR enzymes, blocking them from using natural folates.
The more folic acid present within the system, the slower folate metabolism becomes. If the system becomes saturated with folic acid, some research suggests DHFR enzymes can become completely blocked. A building up of folic acid begins, causing the folate cycle to become out of balance. Anytime an important biochemical process within the body becomes out of sync with its natural cycles there are negative health consequences. If you are trying to become pregnant or are experiencing treatment-resistant health issues, bringing the folate cycle back into its natural cycle is important for ensuring a healthy pregnancy and restoring the body’s methylation system. For a more in-depth article on the folate cycle read our article “The Folate Cycle In Detail”
Changing Your Relationship To Folate
There are major differences between types of folates. Keeping this in mind when choosing what to eat throughout the day. Avoiding foods fortified with folic acid is a good place to start. Unfortunately, folic acid fortification is mandatory in 53 countries around the world. Food such as bread, baked good, cereals are made with enriched wheat and are going to be fortified with folic acid. Making the choice to substitute folate fortified food for food containing natural folates can make a big difference in maintaining a healthy and functioning folate cycle.
Make sure you are including more dark leafy greens in your diet. Refer to our folate containing foods list to get a general sense of what contains folate when planning out your meals. MTHFR mutations decrease your ability to process folate. If you have an MTHFR mutation, avoiding foods fortified with folic acid and getting your natural folates into your diet should be a priority for you. If your health conditions persist after improving your dietary folate intake, you should speak with your healthcare practitioner about supplementing with a combination of active folates. Your situation may be more complex, requiring a specific supplementation strategy to restore a natural and healthy folate cycle.
You can join Carolyn for her next FREE webinar on “What is MTHFR?” for more information about MTHFR and folate. Watch our Events page for updates!
Take home message
The key message here is that we should avoid folic acid where possible, particularly in supplement form and fortified foods, particularly if you have an MTHFR gene mutation. You should be getting as much folate as you can from your diet in the way of leafy green vegetables, and if you have health conditions speak to your practitioner about supplementing with various combinations of active folates ie: folinic acid or 5-MTHF.
References
[1] Aarabi, M., San Gabriel, M. C., Chan, D., Behan, N. A., Caron, M., Pastinen, T., … & Trasler, J. (2015). High-dose folic acid supplementation alters the human sperm methylome and is influenced by the MTHFR C677T polymorphism. Human molecular genetics, 24(22), 6301-6313.
[2]Hekmatdoost, A., Vahid, F., Yari, Z., Sadeghi, M., Eini-Zinab, H., Lakpour, N., & Arefi, S. (2015). Methyltetrahydrofolate vs Folic Acid Supplementation in Idiopathic Recurrent Miscarriage with Respect to Methylenetetrahydrofolate Reductase C677T and A1298C Polymorphisms: A Randomized Controlled Trial. PloS one, 10(12), e0143569.
[3] https://www.cdc.gov/ncbddd/folicacid/recommendations.html
[4] https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/
[5] Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. (1998). Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. National Academies Press (US).
[6] Jacques, P. F., Bostom, A. G., Williams, R. R., Ellison, R. C., Eckfeldt, J. H., Rosenberg, I. H., … & Rozen, R. (1996). Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation, 93(1), 7-9.
Low Homocysteine Levels: What are The Consequences?
What Is Homocysteine?
Homocysteine is an amino acid derivative that serves as an intermediate in the synthesis of methionine and cysteine. Low homocysteine develops when methionine, cysteine and folate levels become disrupted. Homocysteine contains a sulfhydryl group that serves as an important branch in the formation of important biological compounds such as glutathione (GSH) and S-adenosylmethionine (SAM). GSH is formed through the transsulfuration (TS) pathway with the formation of cysteine from homocysteine by a stepwise process by the enzymes cystathionine-beta-synthase (CBS) and cystathionine-gamma-lyase (CGL), and by subsequent formation of GSH by glutathione synthetase. SAM is formed by methylation of homocysteine to form methionine by the enzyme N5,N10-methylenetetrahydrofolate reductase (MTHFR) and subsequent condensation of methionine with adenosine triphosphate (ATP) to form SAM.
What You Should Know About Low Homocyesteine
Low homocysteine levels, or hypohomocysteinemia, is the result of several factors. It can be a result of a metabolic insult which prompts the body to produce more GSH than normal, low intake of the amino acids methionine and cysteine (methionine predominantly comes from meat protein so those that don’t eat meat or eat too little may be at higher risk), an inherently low level of the enzyme MTHFR, low intake of vitamins folate and B12, or increased detoxification of xenobiotics through the phase II liver reaction sulfation. These factors will result in low homocysteine levels, which would then lead to a variety of clinical disorders.
Hypohomocysteinemia has been shown to play a role in the malnutrition-inflammation-cachexia syndrome with which low dietary intake of methionine would lead to worsening of chronic kidney disease, with which homocysteine can be a variable in determining survivability of the patient. Hypohomocysteinemia is also a cause of the progression of atherosclerosis in cardiovascular diseases.
Since hypohomocysteinemia may also present with a lack of cysteine levels, it can be due to the role that the liver plays. Synthesis of GSH requires cysteine, in which the formation of GSH will get homocysteine from the reservoir to support the body in times of oxidative stress, in which GSH may be able to mediate and prevent oxidative damage to cells. The synthesis of GSH would, therefore, favor the TS pathway. So basically this means if there is a low level of antioxidants, the body will steal cysteine from homocysteine to support glutathione production. So low homocysteine may be a sign of oxidative stress.Formation of more cysteine from homocysteine would then deplete the homocysteine pool to support the formation of GSH to combat the free radicals.
Another factor that would deplete homocysteine levels would be the detoxification pathway sulfation. Sulfation is a phase II or conjugation reaction in which cysteine donates a sulfur group to the xenobiotic forming a xenobiotic-sulfate, allowing it to be excreted from the system. Apart from sulfation, cysteine is also employed in the formation of bile acids such as taurine. Taurine is especially synthesized when the body takes in fat and alcohol. Synthesis of taurine would also deplete homocysteine levels by promoting the TS pathway in the formation of cysteine. These homocysteine-depleting mechanisms are prevented if there are adequate amounts of methionine and cysteine from the diet.
Low dietary intake of vitamins folate and B12 can also lead to hypohomocysteinemia. Folate is an important component of the methylation pathway as it forms the structure of N5,N10-methylenetetrahydrofolate and would, therefore, be an important substrate for the transmethylation pathway involving the formation of SAM by transferring a methyl group from N5,N10-methylenetetrahydrofolate to homocysteine forming methionine, catalyzed by the enzyme MTHFR. Folate is important in regulating gene expression by regulating levels of SAM.3
Vitamin B12 is another vitamin that is important in gene expression and DNA synthesis. Methylcobalamin which serves as a cofactor in the methylation cycle is involved in the production of methionine from homocysteine by the action of MTHFR. Low dietary intake of either or both folate and vitamin B12 will result in low methionine levels, which would ultimately inhibit the formation of homocysteine.4
MTHFR deficiency may also lead to low homocysteine levels in the body. MTHFR is the enzyme responsible for the transfer of a methyl group from N5,N10-methylenetetrahydrofolate to homocysteine forming methionine. Defects in the gene, most notably the C677T gene polymorphism, may result in low 5-MTHF levels, resulting in hypohomocysteinemia.5
The effects of having hypohomocysteinemia are mainly due to the prolonged depletion of cysteine. That said, the effects of hypohomocysteinemia would be the prolonged inflammatory status experienced by the body due to the increased formation of free radicals with the lack of GSH to counteract the effect. Inflammation can lead to several disorders that may affect the cardiovascular system, nervous system, and even the renal system. Atherosclerosis is a major concern due to the inflammatory nature of the disease. Inflammation will induce the misfolding of the proteins in the brain causing Alzheimer’s disease. Chronic renal failure may result in the increased inflammation of the tubular system. GSH plays a central role in mediating the effects of oxidative stress, preventing damage to the organs. That said, GSH production relies heavily on the pool of homocysteine to fuel its synthesis.
REFERENCES:
- Ganguly, P. & Alam, S.F. Role of homocysteine in the development of cardiovascular disease. USA: Nutrition Journal. January 2015; Vol. 14, No. 6.
- Lord, R. & Fitzgerald, K. Significance of Low Plasma Homocysteine. USA: Metametrix Laboratory Department of Science and Education. 2006. Retrieved https://www.drkendalstewart.com/wp-content/uploads/2011/09/Significance-of-Low-Plasma-Homocysteine.pdf
- Blom, H. & Smulders, Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. USA: Journal of Inherited Metabolic Disorders. February 2011; Vol. 34, No. 1. pp. 75-81.
- O’Leary, F. & Samman, S. Vitamin B12 in Health and Disease. USA: Nutrients. March 2010; Vol. 2, No. 3. pp. 299-316.
- Leclerc, D. et al. Molecular Biology of Methylenetetrahydrofolate Reductase (MTHFR) and Overview of Mutations/Polymorphisms. Madame Curie Bioscience Database.